- a Department of Obstetrics and Gynecology, School of Medicine, University of Sao Paulo, Sao Paulo, Brazil
- b Jewish Teaching and Research Institute, Albert Einstein Hospital, Sao Paulo, Brazil
Abstract
Objective
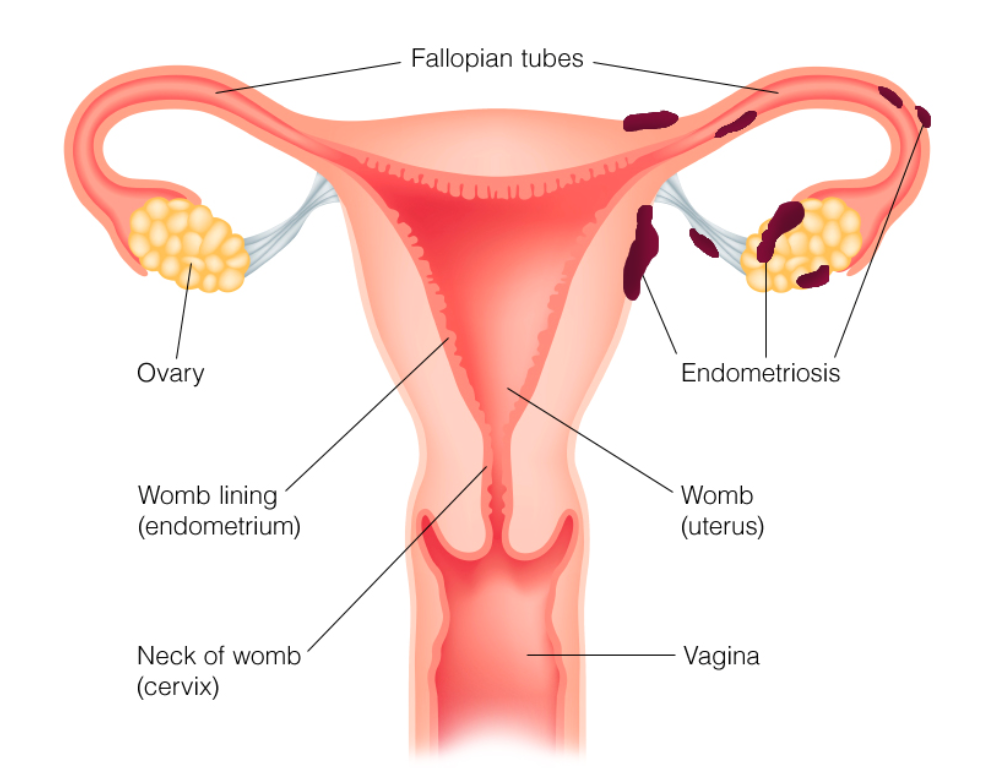
To evaluate the efficacy and safety of potentized estrogen compared to placebo in homeopathic treatment of endometriosis-associated pelvic pain (EAPP).
Study design
The present was a 24-week, randomized, double-blind, placebo-controlled trial that included 50 women aged 18–45 years old with diagnosis of deeply infiltrating endometriosis based on magnetic resonance imaging or transvaginal ultrasound after bowel preparation, and score ≥ 5 on a visual analogue scale (VAS: range 0 to 10) for endometriosis-associated pelvic pain. Potentized estrogen (12cH, 18cH and 24cH) or placebo was administered twice daily per oral route. The primary outcome measure was change in the severity of EAPP global and partial scores (VAS) from baseline to week 24, determined as the difference in the mean score of five modalities of chronic pelvic pain (dysmenorrhea, deep dyspareunia, non-cyclic pelvic pain, cyclic bowel pain and/or cyclic urinary pain). The secondary outcome measures were mean score difference for quality of life assessed with SF-36 Health Survey Questionnaire, depression symptoms on Beck Depression Inventory (BDI), and anxiety symptoms on Beck Anxiety Inventory (BAI).
Results
The EAPP global score (VAS: range 0 to 50) decreased by 12.82 (P < 0.001) in the group treated with potentized estrogen from baseline to week 24. Group that used potentized estrogen also exhibited partial score (VAS: range 0 to 10) reduction in three EAPP modalities: dysmenorrhea (3.28; P < 0.001), non-cyclic pelvic pain (2.71; P = 0.009), and cyclic bowel pain (3.40; P < 0.001). Placebo group did not show any significant changes in EAPP global or partial scores. In addition, the potentized estrogen group showed significant improvement in three of eight SF-36 domains (bodily pain, vitality and mental health) and depression symptoms (BDI). Placebo group showed no significant improvement in this regard. These results demonstrate superiority of potentized estrogen over placebo. Few adverse events were associated with potentized estrogen.
Conclusions
Potentized estrogen (12cH, 18cH and 24cH) at a dose of 3 drops twice daily for 24 weeks was significantly more effective than placebo for reducing endometriosis-associated pelvic pain.
Trial registration: ClinicalTrials.gov Identifier: NCT02427386.
Keywords
- Homeopathy;
- Endometriosis;
- Pelvic pain;
- Homeopathic remedy;
- Rebound effect;
- Placebo;
- Randomized controlled trial (RCT)
- homeopathic treatment endometriosis
- Alternative treatment for endometriosis
- pain of endometriosis